Basic Info
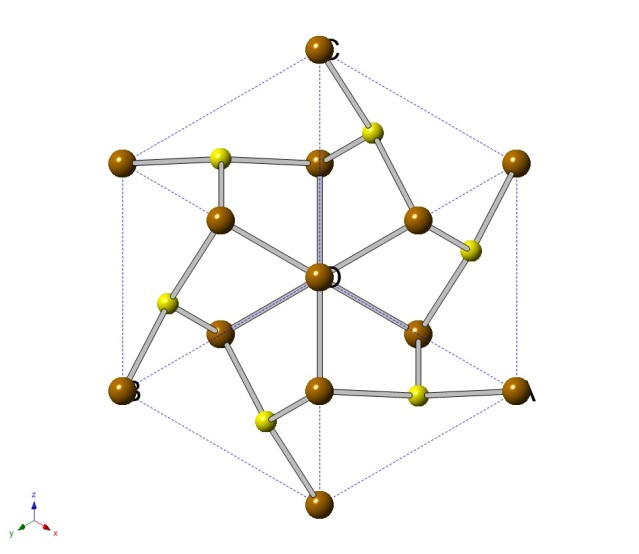
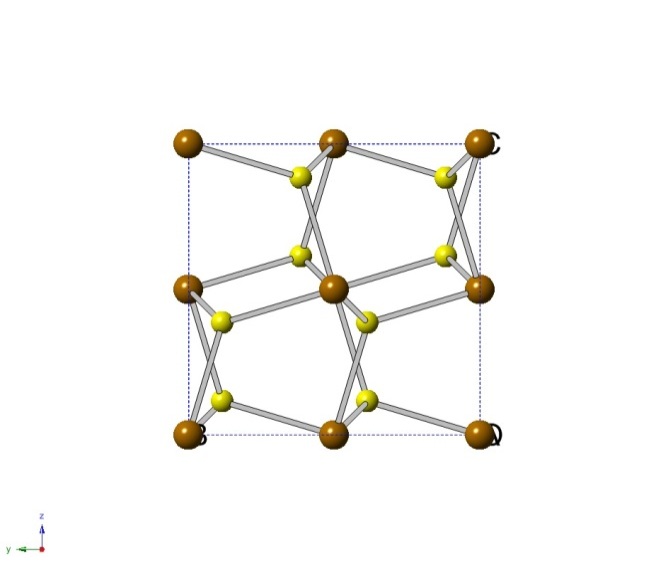
Pyrite, formally known as Iron disulfide, is the most abundant naturally occurring of the sulfide minerals. It has a crystal structure that resembles the fluorite structure. Iron disulfide has a yellow-brass, metallic luster that is sometimes incorrectly recognized as gold. Due to this mistaken identity it is often referred to as “fool’s gold”. [5]
As the result of sparks generated when struck against metal, pyrite was used as a source of ignition for early firearms. Pyrite is also used for commercial production of sulfur dioxide, which is used in the paper industry as well as in the manufacture of sulfuric acid. Fool’s gold also has applications in jewelry, mineral detection in radio receivers, and photovoltaics. [5]
Crystal Structure
| Fractional Coordinates | Orthogonal Coordinates | |||||||
|---|---|---|---|---|---|---|---|---|
| Label | Elmt | x | y | z | xor[Å] | yor[Å] | zor[Å] | |
| 1. | Fe | 0.0000 | 0.0000 | 0.0000 | 0.000 | 0.000 | 0.000 | |
| 2. | Fe | 0.5000 | 0.0000 | 0.5000 | 2.862 | 0.058 | 2.544 | |
| 3. | Fe | 0.5000 | 0.5000 | 0.0000 | 2.646 | 2.765 | -0.157 | |
| 4. | Fe | 0.0000 | 0.5000 | 0.5000 | 0.102 | 2.709 | 2.705 | |
| 5. | Fe | 1.0000 | 0.0000 | 0.0000 | 5.405 | 0.114 | -0.318 | |
| 6. | Fe | 1.0000 | 0.5000 | 0.5000 | 5.507 | 2.823 | 2.387 | |
| 7. | Fe | 0.0000 | 1.0000 | 0.0000 | -0.114 | 5.415 | 0.004 | |
| 8. | Fe | 0.5000 | 1.0000 | 0.5000 | 2.748 | 5.473 | 2.548 | |
| 9. | Fe | 1.0000 | 1.0000 | 0.0000 | 5.291 | 5.529 | -0.314 | |
| 10. | Fe | 0.0000 | 0.0000 | 1.0000 | 0.318 | 0.003 | 5.407 | |
| 11. | Fe | 0.5000 | 0.5000 | 1.0000 | 2.964 | 2.767 | 5.250 | |
| 12. | Fe | 1.0000 | 0.0000 | 1.0000 | 5.723 | 0.117 | 5.089 | |
| 13. | Fe | 0.0000 | 1.0000 | 1.0000 | 0.204 | 5.417 | 5.411 | |
| 14. | Fe | 1.0000 | 1.0000 | 1.0000 | 5.609 | 5.532 | 5.093 | |
| 15. | S | 0.3849 | 0.3849 | 0.3849 | 2.159 | 2.129 | 1.960 | |
| 16. | S | 0.8849 | 0.3849 | 0.1151 | 4.776 | 2.185 | 0.343 | |
| 17. | S | 0.1151 | 0.8849 | 0.3849 | 0.644 | 4.806 | 2.048 | |
| 18. | S | 0.6151 | 0.8849 | 0.1151 | 3.261 | 4.862 | 0.430 | |
| 19. | S | 0.6151 | 0.6151 | 0.6151 | 3.450 | 3.403 | 3.133 | |
| 20. | S | 0.1151 | 0.6151 | 0.8849 | 0.833 | 3.346 | 4.750 | |
| 21. | S | 0.8849 | 0.1151 | 0.6151 | 4.966 | 0.726 | 3.045 | |
| 22. | S | 0.3849 | 0.1151 | 0.8849 | 2.349 | 0.670 | 4.662 | |
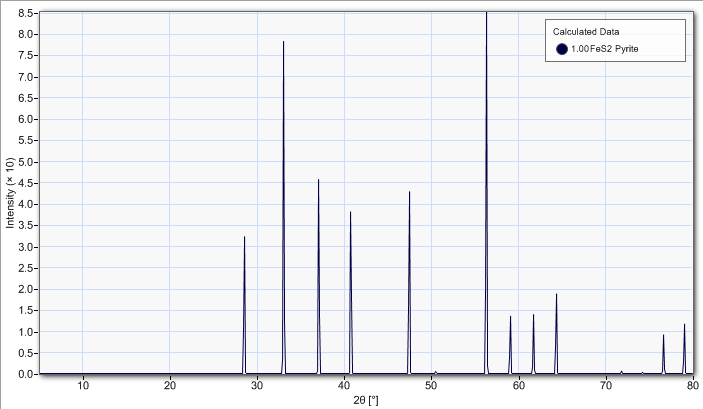
Theoretical diffraction data using a Cu Kα monochromatic source.
(m is the multiplicity and N is the maximum number of flexions)
| ref no. | h | k | l | d(hkl) | 2-Theta | Intensity | I/Imax | m | N |
|---|---|---|---|---|---|---|---|---|---|
| [ 1] | 1 | 1 | 1 | 3.12693 | 28.5207 | 3.45405e-002 | 37.7 | 8 | 3 |
| [ 2] | 0 | 0 | 2 | 2.70800 | 33.0502 | 8.38185e-002 | 91.5 | 6 | 4 |
| [ 3] | 0 | 2 | 1 | 2.42211 | 37.0850 | 4.90083e-002 | 53.5 | 12 | 5 |
| [ 4] | 1 | 1 | 2 | 2.21107 | 40.7741 | 4.07521e-002 | 44.5 | 24 | 6 |
| [ 5] | 0 | 2 | 2 | 1.91485 | 47.4381 | 4.57922e-002 | 50.0 | 12 | 8 |
| [ 6] | 1 | 2 | 2 | 1.80533 | 50.5106 | 5.56486e-004 | 0.6 | 24 | 9 |
| [ 7] | 1 | 1 | 3 | 1.63299 | 56.2871 | 9.15808e-002 | 100.0 | 24 | 11 |
| [ 8] | 2 | 2 | 2 | 1.56346 | 59.0306 | 1.45073e-002 | 15.8 | 8 | 12 |
| [ 9] | 0 | 2 | 3 | 1.50213 | 61.6974 | 1.49215e-002 | 16.3 | 12 | 13 |
| [10] | 1 | 2 | 3 | 1.44749 | 64.2989 | 2.01434e-002 | 22.0 | 48 | 14 |
| [11] | 0 | 0 | 4 | 1.35400 | 69.3429 | 3.85639e-005 | 0.0 | 6 | 16 |
| [12] | 0 | 4 | 1 | 1.31357 | 71.8008 | 6.67362e-004 | 0.7 | 36 | 17 |
| [13] | 1 | 1 | 4 | 1.27656 | 74.2244 | 3.85281e-004 | 0.4 | 24 | 18 |
| [14] | 1 | 3 | 3 | 1.24252 | 76.6194 | 9.79268e-003 | 10.7 | 24 | 19 |
| [15] | 0 | 2 | 4 | 1.21105 | 78.9908 | 1.25237e-002 | 13.7 | 24 | 20 |
| [16] | 1 | 2 | 4 | 1.18187 | 81.3432 | 8.38154e-003 | 9.2 | 48 | 21 |
| [17] | 2 | 3 | 3 | 1.15470 | 83.6810 | 4.14859e-003 | 4.5 | 24 | 22 |
| [18] | 2 | 2 | 4 | 1.10554 | 88.3290 | 1.21383e-002 | 13.3 | 24 | 24 |
| [19] | 0 | 4 | 3 | 1.08320 | 90.6469 | 3.45885e-004 | 0.4 | 12 | 25 |
| [20] | 1 | 3 | 4 | 1.06217 | 92.9660 | 5.11313e-004 | 0.6 | 48 | 26 |
| [21] | 1 | 1 | 5 | 1.04231 | 95.2899 | 3.31754e-002 | 36.2 | 32 | 27 |
| [22] | 0 | 2 | 5 | 1.00573 | 99.9680 | 1.10694e-002 | 12.1 | 60 | 29 |
PV Applications
The primary method of pyrite for photovoltaic applications is that of thin-films. It demonstrates extremely promising results for the use as the active layer in solar photovoltaic and photoelectrochemical cells. Pyrite has a suitable band gap (Eg = 0.95 eV), effective light absorption (R > 105 cm-1 for hν > 1.3 eV), an adequate minority carrier diffusion length (100-1000 nm), and for all intensive purposes is in infinite elemental abundance. In principle, all of U.S. primary power demand (∼3.5 TW) could be met with 10% of the pyrite that is disposed annually as mining waste in six U.S. states alone (assuming 10% cell efficiency and a conservative 5 μm thick pyrite active layer). [6]
Basic Parameters at 300 K
| Crystal structure: | Flourite | [2] |
| Group of symmetry: | Pa(-3) | [2] |
| Number of atoms in 1 cm3: | 7.55*1022 | [2] |
| Unit cell volume: | 158.8678 Å3 | [2] |
| Atoms per unit cell: | 12 | [2] |
| Auger recombination coefficient C: | 10-26 cm6 s-1 | [2] |
| Debye temperature: | 6*106 K | [8] |
| Density: | 5.0159 g/cm3 | [2] |
| Dielectric constant: | ɛ=10.9 | [3] |
| Effective electron density: | Nc = 3*1018 cm-3 | [3] |
| Effective electron masses: | me* = 0.25me | [3] |
| Effective hole density: | Nv = 3*1019 ± 5*1019 cm-3 | [3] |
| Effective hole masses: | mh* = (2.2 ± 0.7) me | [3] |
| Lattice constant: | 5.416 Å | [1] |
| Optical phonon energy: | 1.048 ± 0.005 eV | [8] |
Band structure and carrier concentration
Band Structure and carrier concentration
Graph on the amount of photo-generated carriers as a function of thickness of the planar pyrite and silicon film may be found in Pietro P. Altermatt, Tobias Kiesewetter, Klaus Ellmer, Helmut Tributsch, Specifying targets of future research in photovoltaic devices containing pyrite (FeS2) by numerical modelling, Solar Energy Materials and Solar Cells, Volume 71, Issue 2, 1 February 2002, Pages 181-195, ISSN 0927-0248, 10.1016/S0927-0248(01)00053-8., respectively, neglecting reflection [3]
Graph of Majority carrier mobility as a function of majority carrier density of natural and synthetic pyrite crystals and of pyrite thin films can be found in Pietro P. Altermatt, Tobias Kiesewetter, Klaus Ellmer, Helmut Tributsch, Specifying targets of future research in photovoltaic devices containing pyrite (FeS2) by numerical modelling, Solar Energy Materials and Solar Cells, Volume 71, Issue 2, 1 February 2002, Pages 181-195, ISSN 0927-0248, 10.1016/S0927-0248(01)00053-8.[3]
Graph of the lifetime of excess carriers as a function of majority carrier density for vaiour Auger coefficients C can be found in Pietro P. Altermatt, Tobias Kiesewetter, Klaus Ellmer, Helmut Tributsch, Specifying targets of future research in photovoltaic devices containing pyrite (FeS2) by numerical modelling, Solar Energy Materials and Solar Cells, Volume 71, Issue 2, 1 February 2002, Pages 181-195, ISSN 0927-0248, 10.1016/S0927-0248(01)00053-8. [3]
Temperature Dependences
Graph of optical absorption edge as a function of temperature may be found in C de las Heras, I J Ferrer and C Sanchez, Temerature dependence of the optical absorption edge of pyrite FeS2 thin films, Departamento de Fisica de Materiales, C-IV, Facultad de Ciencias, Universidad Automona de Madrid, 28049-Madrid, Spain [8]
Donors and Acceptors
Donors: Ni, Co [5]
Acceptors: As [5]
Electrical Properties
Basic Parameters of Electrical Properties
Energy gap: 0.95 eV [1]
Energy spin-orbital splitting: 1.2 eV [11]
Intrinsic carrier concentration: 2.78*1012 cm-3 [3]
Carrier mobility: 120 cm2 V-1 s-1 [8]
Intrinsic resistivity: 0.18 Ω·cm [8]
Mobility and Hall Effect
Mobility parameters: µmax = 300 cm2 / V s [3]
µmin = 0.02 cm2 / V s [3]
cref = 6*1017 cm-3 [3]
β = 1.3 [3]
Recombination Parameters
Optical properties
Refractive index nref = 4.5 on average [3]
Absorption coefficient 5*105 cm-1 (λ<750nm) [3]
Thermal properties
Mechanical properties, elastic constants, lattice vibrations
Basic Parameters
Bulk modulus: 143 GPa [1]
Density: 5.0159 g/cm3 [2]
Hardness: 6.3 on the Mohs scale [7]
Surface microhardness (using Knoop's pyramid test): 792 kg/mm2 @ 100 Gms load [10]
577 kg/mm2 @ 300 Gms load [10]
Cleavage planes: (1 1 0), (1 1 1), (0 0 1) [9]
Elastic Constants
Elastic Constants: C11 = 3.46-3.818 Mbars [9]
C12 = -0.529-0.34 Mbars [9]
C44 = 0.68-1.187 Mbars [9]
Data on Raman spectrum of polycrystalline thin film may be found in C de las Heras, I J Ferrer and C Sanchez, Temerature dependence of the optical absorption edge of pyrite FeS2 thin films, Departamento de Fisica de Materiales, C-IV, Facultad de Ciencias, Universidad Automona de Madrid, 28049-Madrid, Spain [8]
References
[1] Joseph Muscat, Andrew Hung, Salvy Russo, and Irene Yarovsky CSIRO Minerals, Bayview Avenue, Clayton South, Victoria, 3169, Australia RMIT University, GPO Box 2476V, Melbourne 3001, Victoria, Australia
(http://prb.aps.org/pdf/PRB/v65/i5/e054107)
[2] Rieder M, Crelling J C, Sustai O, Drabek M, Weiss Z, Klementova M
International Journal of Coal Geology 71 (2007) 115-121
Arsenic in iron disulfides in a brown coal from the North Bohemian Basin,
Czech Republic
( http://rruff.geo.arizona.edu/AMS/result.php)
[3] Pietro P. Altermatt, Tobias Kiesewetter, Klaus Ellmer, Helmut Tributsch, Specifying targets of future research in photovoltaic devices containing pyrite (FeS2) by numerical modelling, Solar Energy Materials and Solar Cells, Volume 71, Issue 2, 1 February 2002, Pages 181-195, ISSN 0927-0248, 10.1016/S0927-0248(01)00053-8.
(http://www.sciencedirect.com/science/article/pii/S0927024801000538)
[4] B. Thomas, K. Ellmer, W. Bohne, J. Röhrich, M. Kunst, H. Tributsch, Photoeffects in cobalt doped pyrite (FeS2) films, Solid State Communications, Volume 111, Issue 5, 2 July 1999, Pages 235-240, ISSN 0038-1098, 10.1016/S0038-1098(99)00213-6.
(http://www.sciencedirect.com/science/article/pii/S0038109899002136)
[5] Electrochemical Impedance Spectroscopy of Synthetic Pyrite Doped with As, Co, and Ni
Stephen Lehner, Madalina Ciobanu, Kaye Savage, and David E. Cliffel, J. Electrochem. Soc. 155, P61 (2008), DOI:10.1149/1.2885103
(http://scitation.aip.org/getpdf/servlet/GetPDFServlet?filetype=pdf&id=JE...)
[6] Colloidal Iron Pyrite (FeS2) Nanocrystal Inks for Thin-Film Photovoltaics James Puthussery, Sean Seefeld, Nicholas Berry, Markelle Gibbs, and Matt Law Journal of the American Chemical Society 2011 133 (4), 716-719
(http://pubs.acs.org/doi/pdf/10.1021/ja1096368)
[7] W.A Wooster, Physical Properties and Atomic Arrangements in Crystals, Department of Mineralogy and petrology, University of Cabridge, 1953 Rep. Prog. Phys. 16 62.
( http://iopscience.iop.org/0034-4885/16/1/302/pdf/0034-4885_16_1_302.pdf)
[8] C de las Heras, I J Ferrer and C Sanchez, Temerature dependence of the optical absorption edge of pyrite FeS2 thin films, Departamento de Fisica de Materiales, C-IV, Facultad de Ciencias, Universidad Automona de Madrid, 28049-Madrid, Spain
(http://iopscience.iop.org/0953-8984/6/46/033/pdf/0953-8984_6_46_033.pdf)
[9] Gene Simmons and Francis Birch, Elastic Constants of Pyrite, Dunbar Laboratory, Harvard University, Cambridge, Massachusetts, 1 April 1963.
[10] Forbes Robertson, Knoop Hardness Numbers for 127 Opaque Minerals, Geological Society of American Bulletin 1961;72, no 4;621-637 doi: 10.1130/0016-7606(1961)72[621:KHNFOM]2.0.CO;2.
(http://gsabulletin.gsapubs.org/content/72/4/621.full.pdf)
[11] Surfactant-Assisted Hydrothermal Synthesis of Single phase Pyrite FeS2 NanocrystalsCyrus Wadia, Yue Wu, Sheraz Gul, Steven K. Volkman, Jinghua Guo, and A. Paul Alivisatos Chemistry of Materials 2009 21 (13), 2568-2570.
(http://pubs.acs.org/doi/full/10.1021/cm901273v)
The development of these pages on photovoltaic materials’ properties was carried out at the University of Utah primarily by undergraduate students Jeff Provost and Carina Hahn working with Prof. Mike Scarpulla. Caitlin Arndt, Christian Robert, Katie Furse, Jash Sayani, and Liz Lund also contributed. The work was fully supported by the US National Science Foundation under the Materials World Network program award 1008302. These pages are a work in progress and we solicit input from knowledgeable parties around the world for more accurate or additional information. Contact earthabundantpv@eng.utah.edu with such suggestions. Neither the University of Utah nor the NSF guarantee the accuracy of these values.

